2019
Interface properties of nanoscale layers. — Our work aimed to study the buried interfaces ex situ in polycrystalline trilayer structures by Mössbauer spectroscopy. In a structurally perfect and chemically sharp layered structure it is only two monolayers of Fe atoms at the interface which “feel the presence of other kind of atoms”, since hyperfine parameters of the Mössbauer atom are basically determined by atoms located in its first two coordination shells even in case of metallic systems. This allows us to reveal differences in width and chemical composition of the bottom and top interfaces of nanoscale Fe layers. For this purpose the Mössbauer parameters of trilayer pairs of equal Fe layer thickness are compared, for example Nb/Fe/Nb and Nb/Fe/Ag pairs. (The layer sequence is given as starting from the bottom, i.e. from the substrate side.) The Mössbauer parameters of the Fe-on-Nb interface can be derived from the Nb/Fe/Ag samples, since the Ag-on-Fe interface is chemically very sharp and the Mössbauer parameters of two monolayers of Fe atoms at the Fe-Ag interface are well known from the literature. The Mössbauer parameters of the Nb-on-Fe interface can be derived from the comparison of the Nb/Fe/Nb and Nb/Fe/Ag sample pairs of equal Fe layer thickness, as the fitted parameters and the intensity of the different components are compared. For example, if the bottom and top interfaces of the Fe layer are similar, then the hyperfine parameters of the Fe-Nb interface components remain unaltered and the intensity of the Nb-Fe interface sub-spectrum is halved when the top Nb layer is replaced by Ag.
In case of Nb/Fe/Nb vacuum evaporated trilayers on top of Si(111) substrate our Mössbauer analysis indicated that the chemical mixing forms less than 1 nm thick Fe-rich interfaces at both sides of the Fe layer. It is in accordance with our x-ray reflectometry and Rutherford backscattering spectrometry measurements [1], where the evaluations provided slightly larger - around 2 nm wide and symmetric - interface layers indicating that chemical mixing and geometrical roughness equally contribute to the element distribution across the layers.
 |
 |
| a.) | b.) |
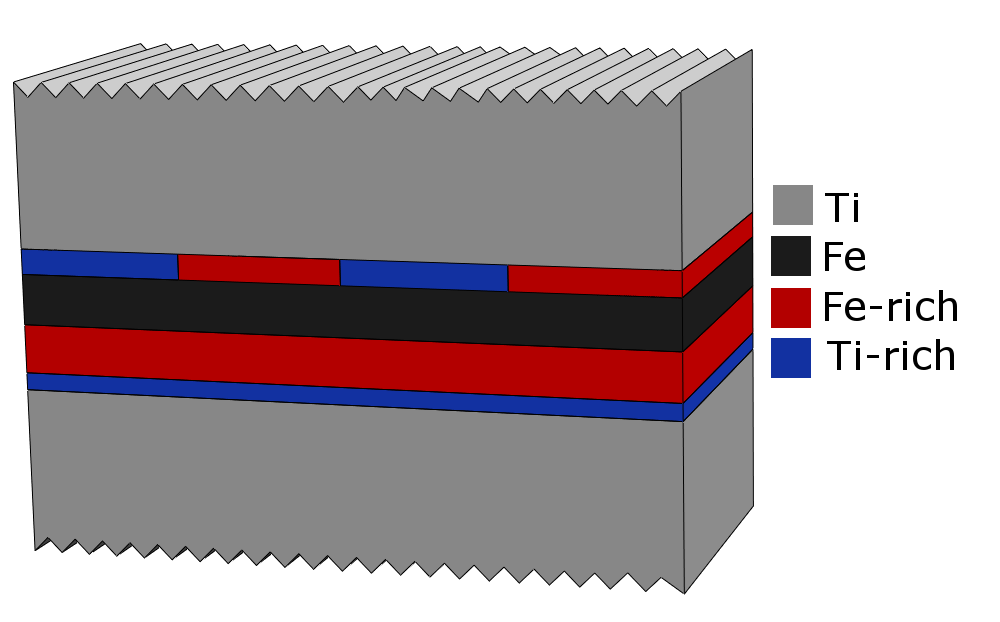
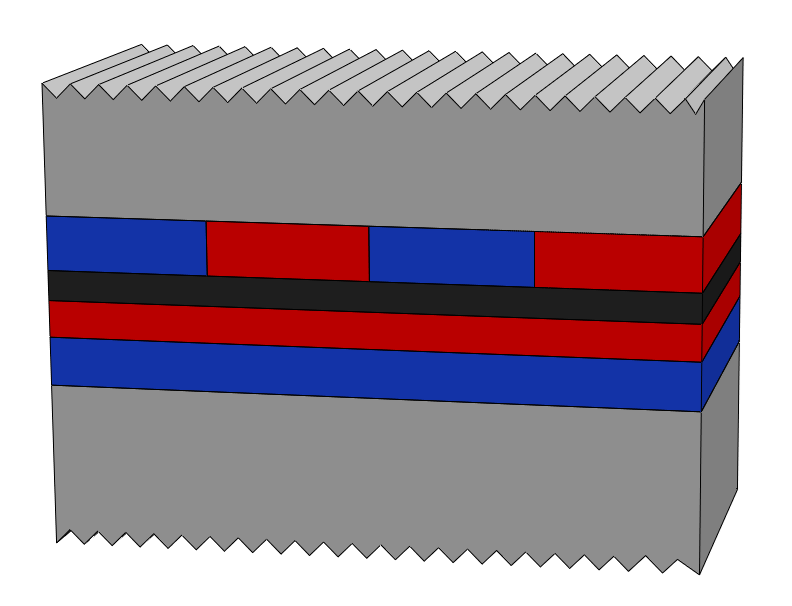
Figure 1. Schematic structure of the interfaces of Ti/Fe/Ti trilayers in the as-received state (a) and after annealing for 2 hours at 250 °C (b). The color code of the Ti, Fe, Fe-rich magnetic alloy and Ti-rich paramagnetic alloy components is shown in the middle.
The different interface width and alloy composition of the Fe-on-Ti and Ti-on-Fe interfaces was shown to result different evolution of the phase compositions at the two interfaces in Ti/Fe/Ti trilayers (Fig. 1) under heat treatments below 300 °C. At the Ti-on-Fe interface the amount of both the paramagnetic and magnetic alloy components increase, while at the Fe-on-Ti interface the amount of the paramagnetic alloy increases at the expense of the magnetic alloy component. The results are explained by the different thickness of the magnetic alloy at the two interfaces and the consequently different degree of layer formation.
Characterization of protein molecules by 1H NMR — To characterize aqueous protein solutions, a novel thermodynamic method was applied. It is based on the two-component wide line 1H NMR signal and provides direct experimental data on the potential energy surface of protein molecules. The temporal behavior of the wide line 1H NMR signal component coming from mobile hydration water proves clearly that during heating the internal energy changes. The amount of mobile hydration water as a function of temperature gives a melting diagram. Melting diagrams provide special characterization of the proteins.
The usefulness and merits of our approach are demonstrated through four globular structured protein examples . Quantitative conclusions could be deduced about the ratios of the globular (ordered) and the more solvent exposed (disordered) regions of the protein molecules and also about the energy relations of the protein-water interactions. These “globular” proteins are disordered to some definite, though small degree. The extent of disorder ranges from 0.14% to 0.49% in these proteins.
Hydration properties of folded and unfolded/disordered miniproteins were monitored in frozen solution . The amount of mobile water as function of T was found characteristically different for folded, semi-folded and disordered variants. Comparing results of wide-line 1H-NMR and molecular dynamics simulations, it was found that both the amount of mobile water, as well as the miniproteins’ thaw profiles differ significantly as function of the compactness and conformational heterogeneity of their structure.
Parkinson’s disease is connected with abnormal α-synuclein (αS) aggregation. Energetics of potential barriers governing motions of hydration water is examined . Information about the distributions and heights of potential barriers is gained by a thermodynamical approach. The αS monomer contains 33% secondary structure and is more compact than a random coil. αS monomers realize all possible hydrogen bonds. Oligomers are ordered by 66%. Wild type and A53T amyloids show identical, low-level hydration, and are considered as disordered to 75%.



