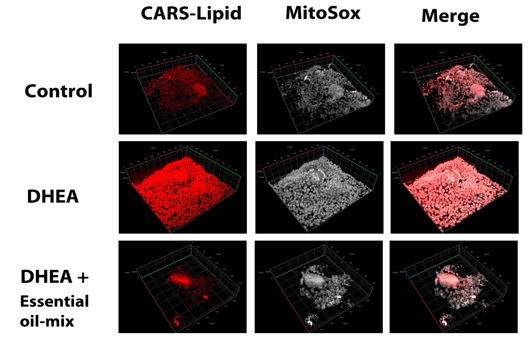
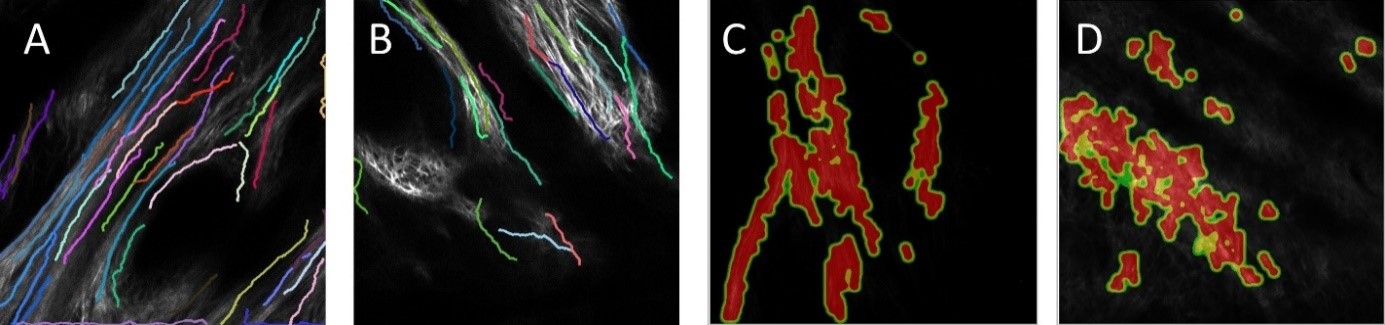
2019
20 MHz repetition rate, sub-picosecond Ti:sapphire laser for high chemical contrast CARS imaging. — Coherent anti-stokes Raman scattering (CARS) microscopy is widely used in label-free biomedical imaging applications. For in vivo diagnostic use of CARS microscopy, wide field detection is preferred to descanned configurations. Chemical selectivity poses a major difficulty when femtosecond (fs) pulse lasers are applied, as their spectral bandwidth is typically significantly higher (~5-10 nm) than the optimum value (~ 1 nm) matching the bandwidth of molecular vibrations. This fact leads to the appearance of an enhanced non-specific background and the decrease of spectral sensitivity in CARS imaging. Last year we proposed a fast spectral modulation technique for sub-100 fs pulse Ti:S lasers , which allowed us to modulate the laser spectrum on ms time scale with the use of a piezo-driven Michelson interferometer. In one of the settings we used, we modulated the laser spectrum of our laser in such a way, that CARS imaging for CH2 bonds in “lipids” and CH3 bonds “proteins” did not require any tuning of the pump (Ti:S) laser or any readjustment of the delay between the pump and Stokes (Yb-amplifier) pulses, which allowed us to record stain-free histological images of brain slices. This year we have developed a ~20 MHz, sub-ps Ti:sapphire laser system, which supports real time, two-channel, high chemical contrast, DVRF CARS imaging, i.e. histology of the skin by a commercial LSM 7 MP microscope (Carl Zeiss, Jena, DE) without any modification of its ZEN software or post-processing of the images like in case of our previous CARS setups used for histology.
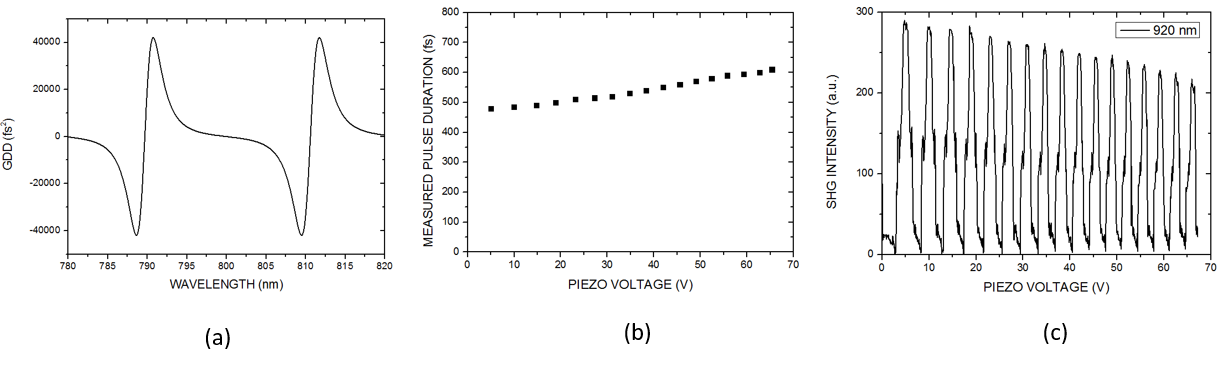
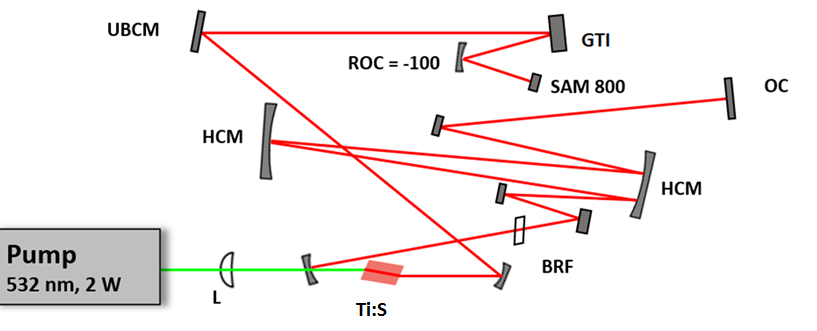
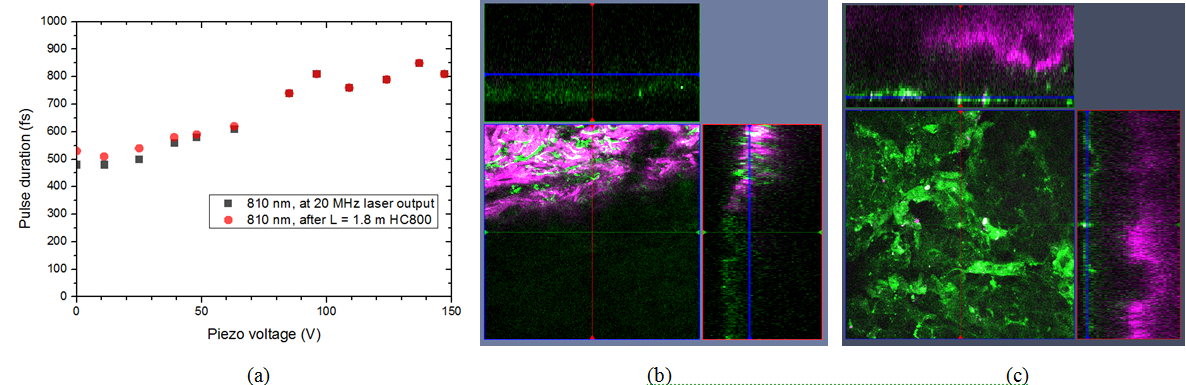
The long cavity laser configuration is similar to that was published in , with a few modifications: 1.) We replaced the SF10 prism pair by a properly designed Gires-Tournois interferometer (GTI, R&D Ultrafast Lasers Ltd.), which provides considerably higher intracavity dispersion than the the prism pair previously used. 2.) Stable mode-locking of the laser is assured by a saturable absorber mirror (SAM, Batop GmbH, DE). 3.) Broad tunability, i.e. the possible use of different SAMs for different molecular vibration regimes is assured by ion-beam sputtered ultrabroadband chirped mirrors (R&D Ultrafast Lasers Ltd). Beside a birefringent tuning element, fine tuning of the Ti:sapphire laser is obtained by the piezo controlled GTI. In our new setup, the spectral bandwidth of the pump (Ti:S) laser has been reduced from 6-8 nm to ~2 nm. Accordingly, the pulse duration has increased from ~150 fs to ~ 600 fs, or slightly above. This four-fold reduction in the peak intensity is compensated by the lower repetition rate of our long cavity Ti:sapphire laser comprising a Herriott-cell and a ~2W average power, 532 nm pump laser. Pulse duration of the ~20 MHz laser has been characterized by a PulseCheck autocorrelator (APE GmbH, DE). Depending on the intracavity dispersion set by the mirror spacing of an intracavity GTI, the pulse duration can be set in the 0.6-1 ps range.
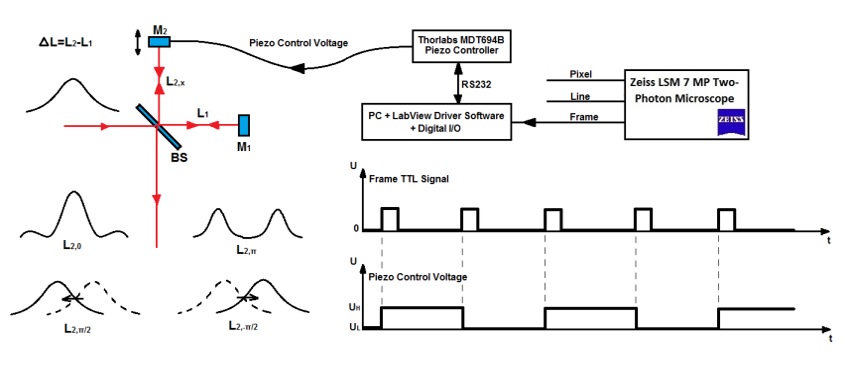
Real time, stain free, high contrast histology of the skin. — The ~20 MHz repetition rate, sub-ps Ti:sapphire (Ti:S) laser introduced in the previous section has been tested for real time, high chemical contrast dual vibration resonance frequency (DVRF) CARS imaging of the skin, which is suitable for in vivo histology. For our comparative studies, we used two different CARS imaging setups, as shown in Fig. 1. In our setup at the University Szeged (USZ), we used a ~80 MHz, ~80 fs Ti:S laser (Mai Tai, Newport Spectra-Physics, USA) as a pump laser (for details, see Refs. and ). In the setup at Wigner RCP, Budapest, we replaced our industrial standard, ~76 MHz, ~150 fs Ti:S laser by our newly developed, ~20 MHz repetition rate, sub-ps Ti:S laser. Spectral bandwidth of the ~20 MHz Ti:sapphire laser (pump) was measuredΔλ < 2 nm allowing high spectral resolution DVRF-CARS imaging. For higher spectral contrast between the anti-Stokes signals generated by „lipids” and „proteins”, we placed a Michelson interferometer similar to that was used in Ref. 1 into the beam path of our Stokes (Yb) laser. By spectral modulation, we obtained a double peaked spectrum with a peak separation of 5-6 nm at around 1030 nm. DVRF CARS imaging was performed by two NDD detectors of our microscope: the anti-Stokes signals for „lipids” and „proteins” were separated by a dichroic mirror with a long pass edge at around 645 nm, while two bandpass filters with central wavelengths at 641 nm and 650 nm were respectively placed in front of the NDD-s. The optical signal detected by the „lipid” detector was pseudo-colored red, while that of the „protein” was given the color blue to match conventional H&E stained histology.
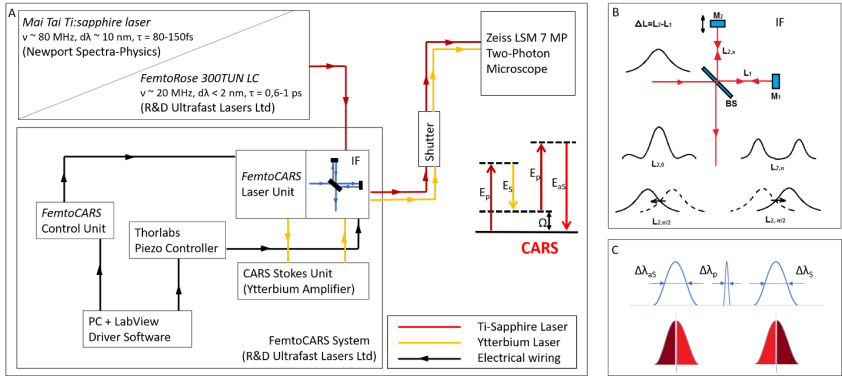
Figure 1. A) Layout of the experimental setups used for IF-CARS and real time DVRF CARS imaging with a 20 MHz, sub-ps Ti:S laser. In both setups, a Michelson-interferometer is used for spectral modulation of either the pump (IF-CARS setup) or Stokes pulses (real time DVRF CARS setup). By electonically modulating the phase difference (Δφ) of the two mirrors, different output spectra can be obtained. B) Phase difference Δφ = 0 at the central frequency results in a narrower spectrum, whereas Δφ = π results in a double-peaked spectrum around the central wavelenght, when the path difference offset, ΔL is properly set. When the phase difference is π/2 or – π/2, two different spectrally shifted laser spectra is obtained with two maxima a few nm-s apart. C) For high chemical contrast, real time DVRF-CARS imaging with two parallel NDD detectors, at least one of the pump or Stokes laser spectral bandwidth has to be small enough (< 2 nm) to create properly distinguishable anti-Stokes signals. If Δλp is small (~2 nm) and ΔλS is broader (~10 nm), nearly half of the Stokes photons (of lower energy) excites the CH2 bonds in lipids, while others (of higher energy) excite the CH3 -bonds in proteins.
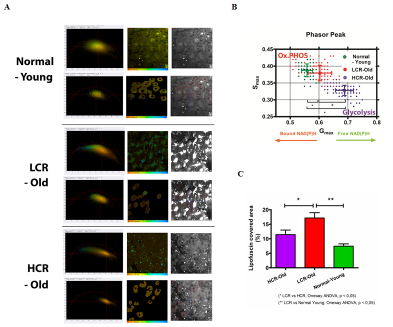
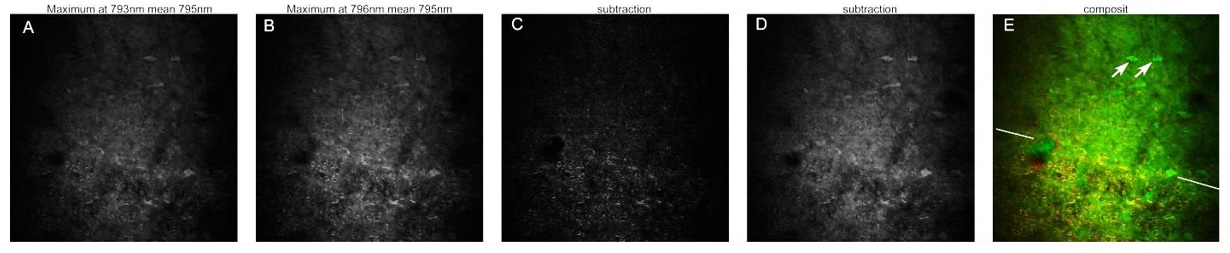
Histological imaging experiments on ex vivo human and murine skin samples by different CARS imaging methods are summarized in Figure2. As a main result, we can say that our new, ~20 MHz, sub-ps Ti:S laser system supports real time, two-channel, high contrast, dual vibration resonance frequency (DVRF) CARS imaging, i.e. histology of the skin by a LSM 7 MP microscope with its original ZEN software, with properly chosen commercial bandpass and dichroic filters in front of the two NDD detectors and without any postprocessing of the images like in case of our previous CARS setups used for histology.
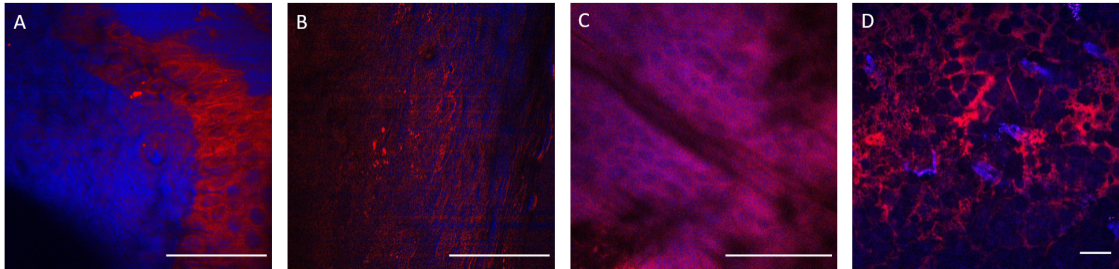
Figure 2. Histological imaging of ex vivo human and murine skin samples by different CARS imaging methods. A) Composite of CARS images recorded for "proteins" and "lipids" in human basal cell cancer (after post-processing) with a Mai Tai pump laser tuned to 790 and 798 nm, respectively. B) IF-CARS imaging of murine skin (after post-processing) with spectrally modulated Mai Tai pump laser pulses with spectral maxima at 792 and 796 nm. C) Post-processed DVRF-CARS image of murine skin with a Mai Tai pump laser tuned to 796 nm with simultaneous detection of the two anti-Stokes signals. D) Real time DVRF-CARS image of murine skin with simultaneous detection of the lipid („-CH2 vibration” ) and protein ("-CH3 vibration") channels with our ~20 MHz sub-ps Ti:sapphire laser tuned to ~795 nm and with a double-peaked (off resonance, see Fig. 1b ) Yb-laser spectrum. Scalebar on each figure: 50 μm.
This new setup can also be used for in vivo experiments on murine skin or ex vivo analysis on human pathological skin (or brain) samples, which, in longer term, may pave the way for applications during Mohs-surgery or real time in vivo diagnosis of skin lesions or brain tumor.
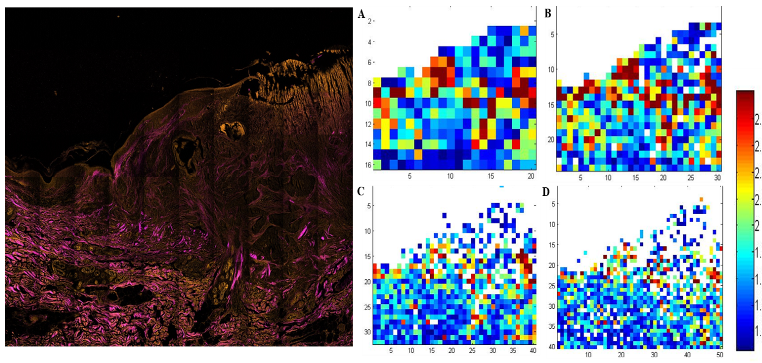
Three-color, stain-free mosaic imaging of Pseudoxanthoma elasticum. — Pseudoxanthoma elasticum is a rare, genetic disease with an autosomal recessive inheritance. PXE is caused by mutations in the ABCC6 gene, which result in decreased serum levels of inorganic pyrophosphate (PPi). As a consequence of low PPi levels, ectopic mineralization and fragmentation of elastic fibers develop in the skin, the endothelium of blood vessels and the Bruch membrane of the eye. PXE is often diagnosed only after severe ophthalmological and cardiovascular complications occur. Multidisciplinary clinical examination, histopathologic evaluation of skin biopsy, in addition to molecular genetic testing is needed for proper diagnosis. Nonlinear microscopy is an innovative non-invasive imaging technology that is increasingly applied in life sciences. Nonlinear processes, such as second-harmonic generation (SHG) or two-photon absorption fluorescence (TPF) generated by ultrafast laser pulses provide stain-free imaging with submicron resolution and higher penetration depth compared to reflectance confocal microscopy. Fibrillar collagen, as being a highly non-centrosymmetric molecule emits strong SHG signal. Elastin fibers and calcium deposits, as endogenous chromophores can be selectively visualized by TPF (see Fig. 3).
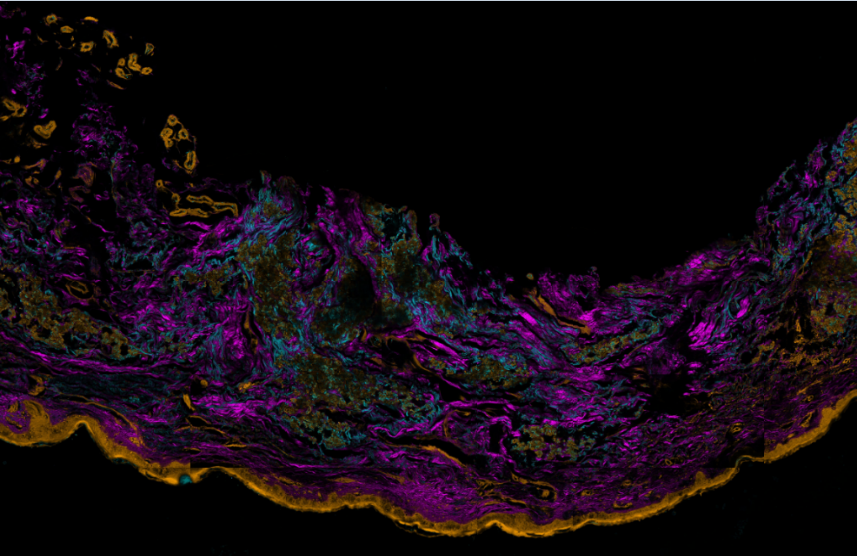
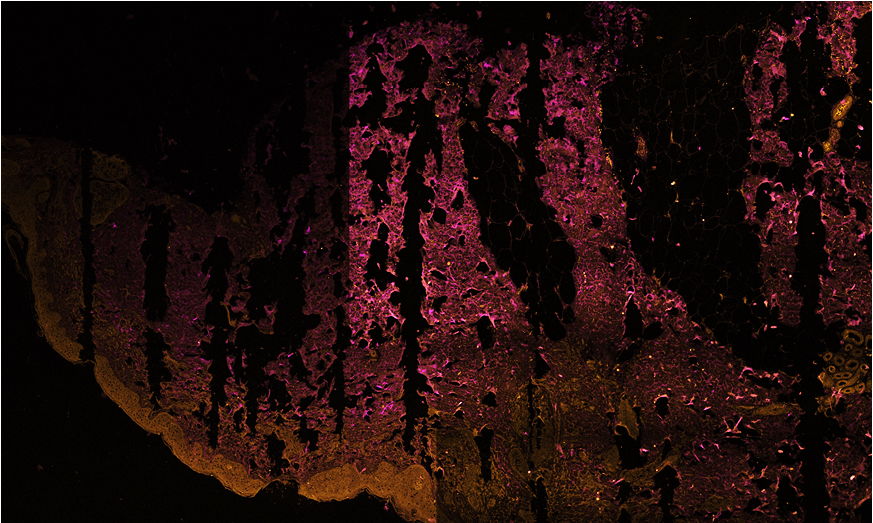
Figure 3. Three color mosaic nonlinear microscope image of a Pseudoxanthoma elasticum skin sample. Collagen, elastin and calcium deposits are respectively visualized in magenta, light blue and yellow colors.
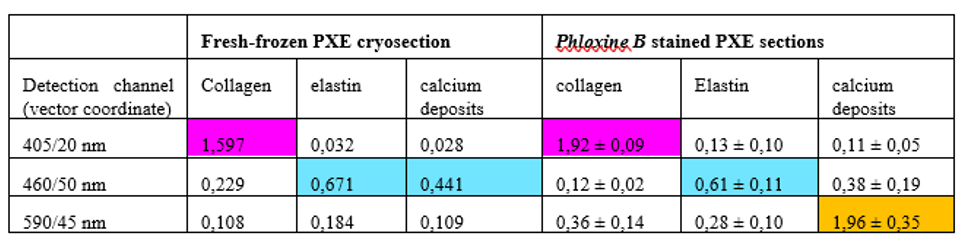
TPF spectra of elastin and calcium deposites overlap considerably, which reduces the chemical contrast in nonlinear microscope imaging. This year we have developed a novel, three-channel detection technique, as well as a numerical image decomposition algorithm for high chemical contrast imaging of collagen, elastin and calcium deposites in PXE samples, the result of which is demonstrated in Figure 3.
Microglia monitor and protect neuronal function via specialized somatic purinergic junctions. — Microglia are the main immune cells in the brain with emerging roles in brain homeostasis and neurological diseases, while mechanisms underlying microglia-neuron communication remain elusive. In a recent experiment, our partners at University Szeged and at the Institute of Experimental Medicine identified a novel site of interaction between neuronal cell bodies and microglial processes in mouse and human brain. Somatic microglia-neuron junctions possess specialized nano-architecture optimized for purinergic signaling. Activity of neuronal mitochondria is linked with microglial junction formation, which is rapidly induced in response to neuronal activation and blocked by inhibition of P2Y12-receptors (P2Y12R). Brain injury-induced changes at somatic junctions trigger P2Y12R-dependent microglial neuroprotection, regulating neuronal calcium load and functional connectivity. Their results suggest that microglial processes at these junctions are in ideal position to monitor and protect neuronal functions in both the healthy and injured brain.
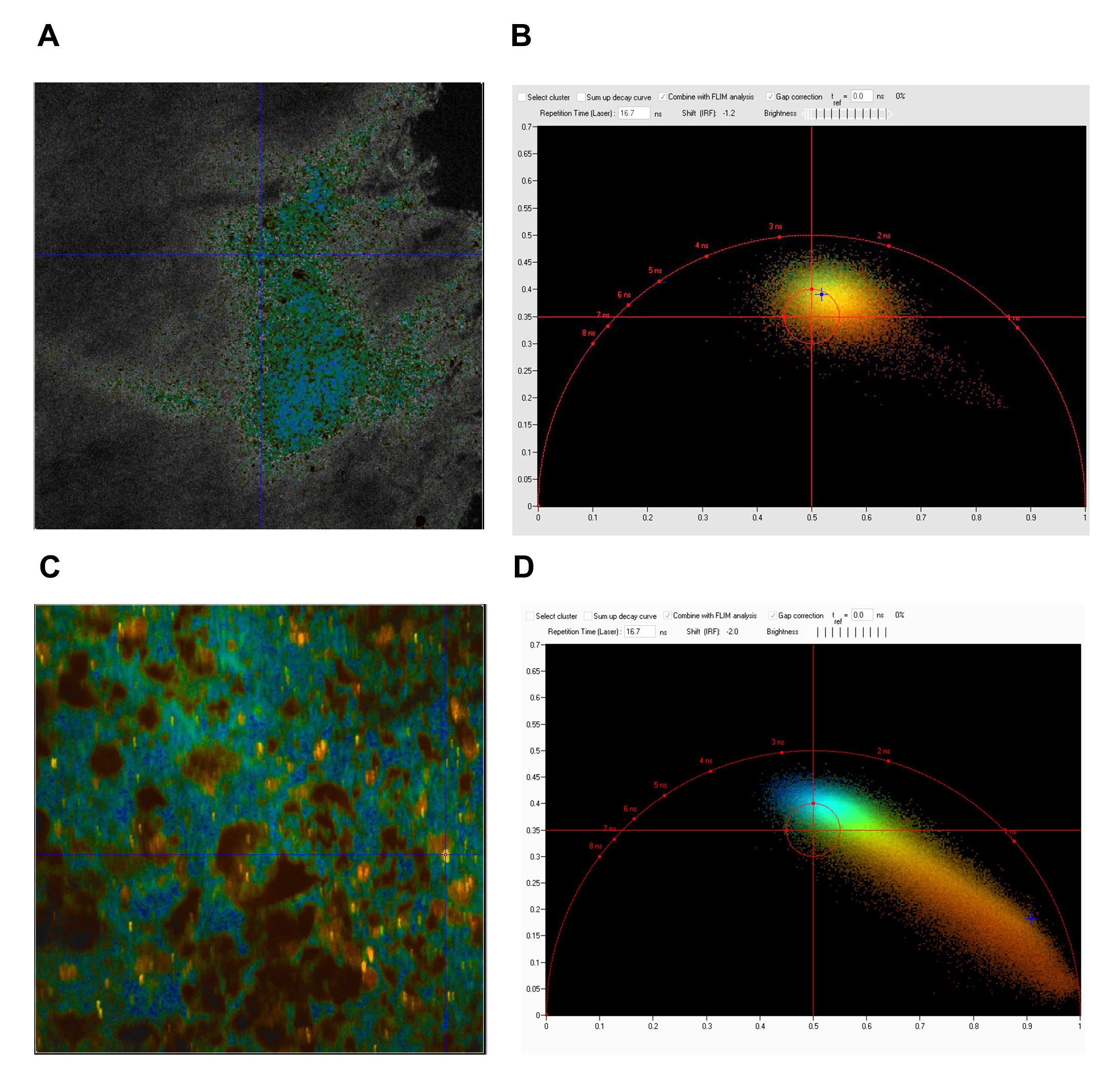
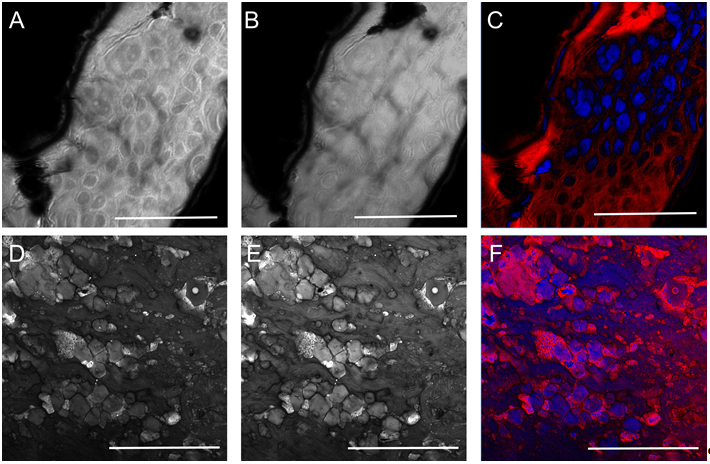
For these real time, stain free two-photon fluorescence imaging experiments performed at University of Szeged, we have developed a laser system comprising two, beam-coupled femtosecond pulse lasers operating at ~750 nm and ~920 nm, respectively. The lasers excited the auto-fluorescence emitted by nicotinamide adenine dinucleotide (NADH) and green fluorescent protein (GFP) simultaneously, which allowed us to record in vivo, time-lapse images of somatic mitochondria in living neurons and microglia, respectively. Representative images of these experiments are shown in Fig. 4. For details, see .
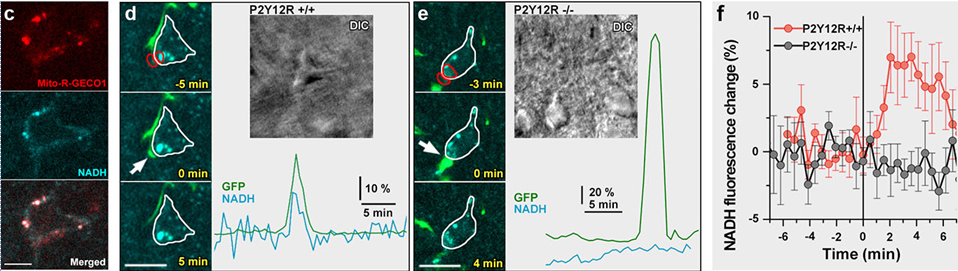
Figure 4. Physiological microglia-neuron communication at the somatic junction site is P2Y12R-dependent and is linked with neuronal mitochondrial activity. a) b) are not shown here. c) Mito-R-Geco1 expression co- nicotinamide adenine dinucleotide (NADH) intrinsic fluorescence. d-e) Representative samples from time-lapse imaging of microglia show processes extend and contact neuronal soma in CX3CR1+/GFP/P2Y12R+/+ (d) and CX3CR1+/GFP/P2Y12R-/- (e) mice. White arrow indicates the contact site of microglia. DIC images of the imaged neurons and the fluorescence signal of GFP (green) and NADH (dark cyan) of red outlined areas are shown. f) Average of NADH intrinsic fluorescence of all neurons in P2Y12R+/+ (red, n=10) and P2Y12R-/- mice (black, n=11).