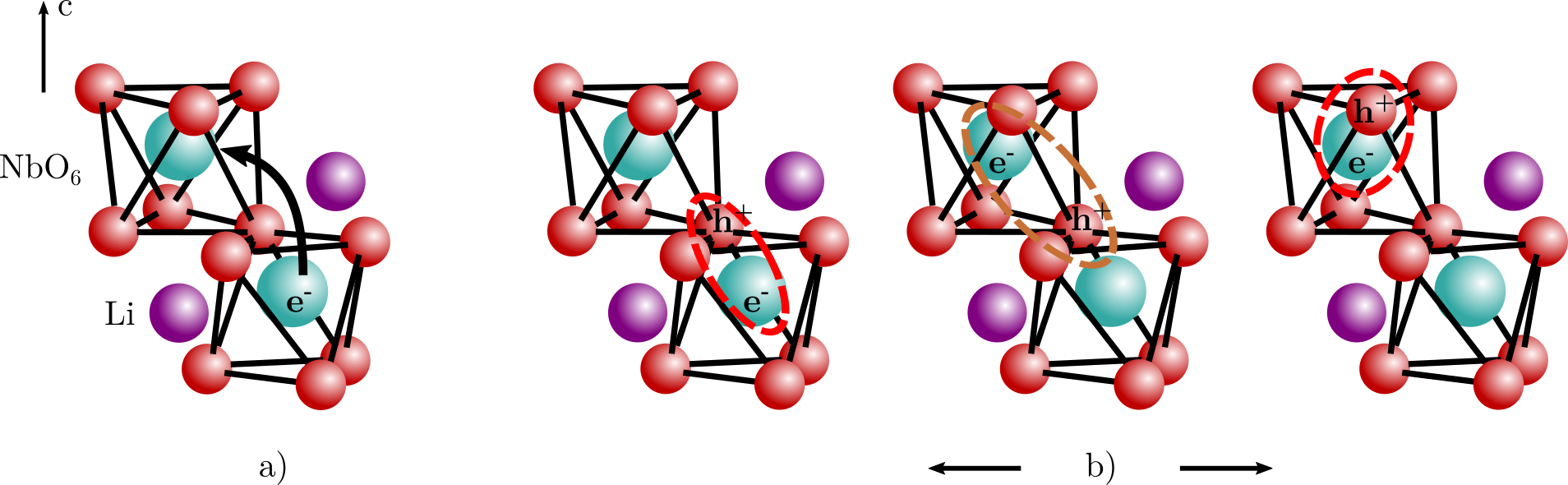2023
Analytical chemical methods for advanced and environmental materials. — High-resolution continuum source atomic absorption spectrometry (HR-CS-AAS) using graphite furnace atomization with solid (powder) and solution sampling were developed and applied to quantitate various dopant elements (Bi, Mg) and impurities (Fe) from microsamples of optical crystals of lithium niobate and raw growth materials. Flame HR-CS-AAS methods were developed with matrix-matching to check the accuracy of the methodology.
Some low-cost sensors (LCSs) were compared for different aerosol size ranges with a research-grade instrument under controlled laboratory conditions. An aerosol generator was utilized to produce various sizes of monodispersed particulate matter (PM), which was introduced into a laboratory smoke chamber under resistance heating/cooling and/or varying RH conditions. Besides, the accuracy of the air temperature (T) and relative humidity (RH) sensors of the LCSs were assessed against calibrated laboratory-grade instruments. The study LCSs showed generally accurate readings for PM2.5, irrespectively of the slow T and/or RH changes, which provided apt conditions for calibration. On the other hand, PM1 and PM10 readings slightly deviated from those observed with the reference monitor, likely due to the lower detection efficacy of the formers towards fine and coarse PM. For T calibration, low RH in the smoke chamber provided more reproducible conditions in terms of the lower measurement bias for LCSs as recorded against a calibrated, reference-grade thermometer.
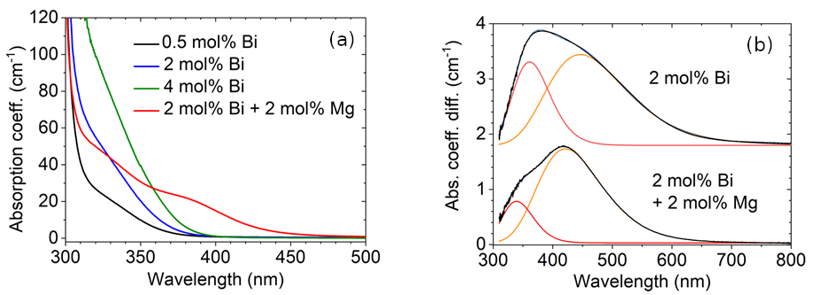
Bi-doped stoichiometric LiNbO3 (SLN) crystals. – A temperature-controlled two-wave mixing setup was built up to investigate photorefractive and photochromic effects in Bi doped, and Bi-Mg co-doped stoichiometric lithium niobate single crystals at 405 nm. Since the second one has been recently shown to be a good candidate for 3D holographic displays, its practical application requires deep study of its photochromic properties besides the photorefractive ones. Beyond long-term photochromic effect we also found a wide time scale of decay components, down to the order of seconds. For this reason, only stretched exponential function can be fitted well to the time evolution of the absorption coefficients. A strong temperature dependence of the nominal time constants was also found with activation energies between 0.3 and 0.7 eV in both LiNbO3:Bi and LiNbO3:Bi,Mg samples. Lower activation energies were found in case of Mg co-doped crystals. Two-wave mixing experiments gave recording time of 25 ms for LiNbO3:Bi,Mg, which is near to the same found in the literature for congruent crystals measured at 442 nm.
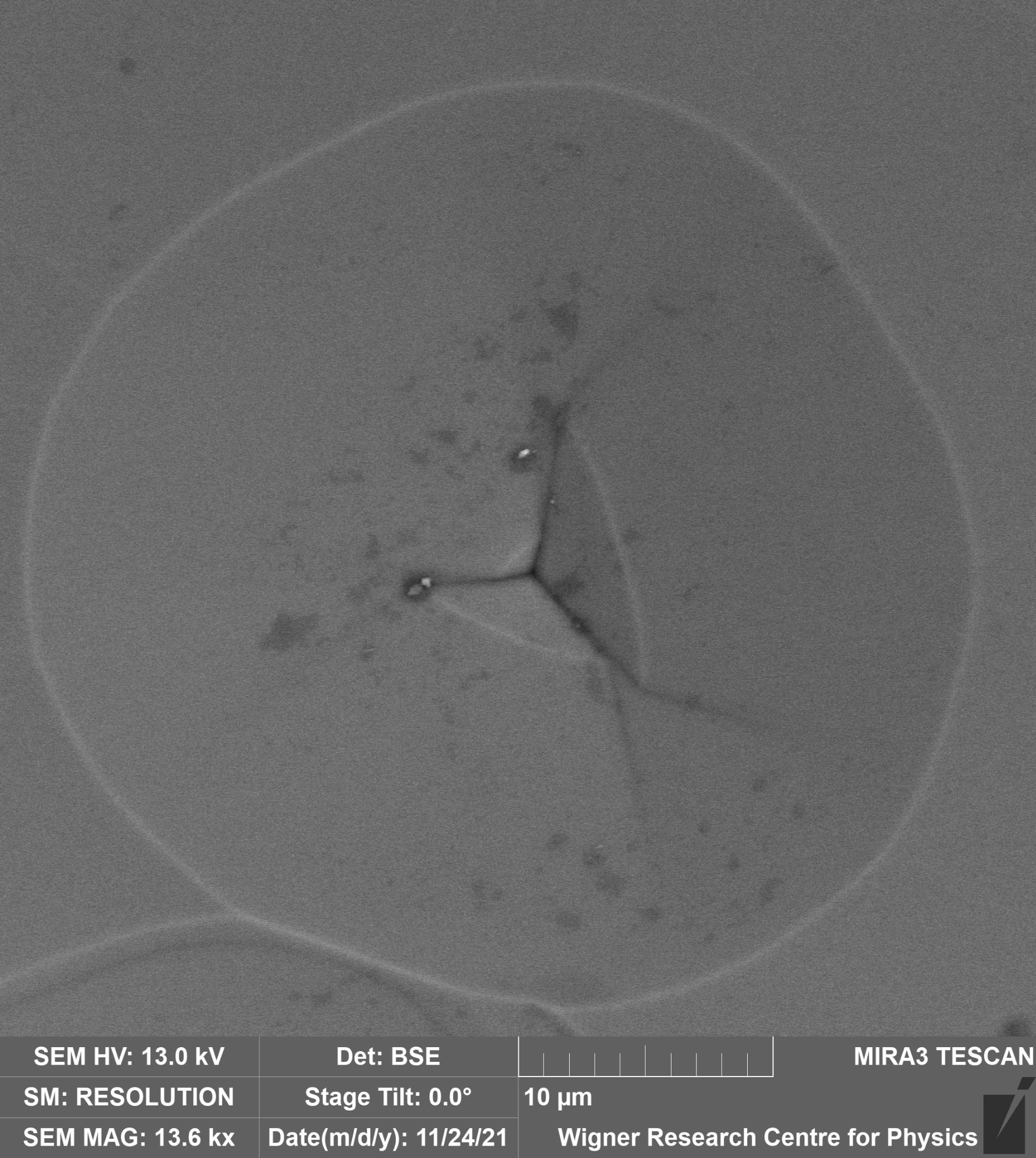
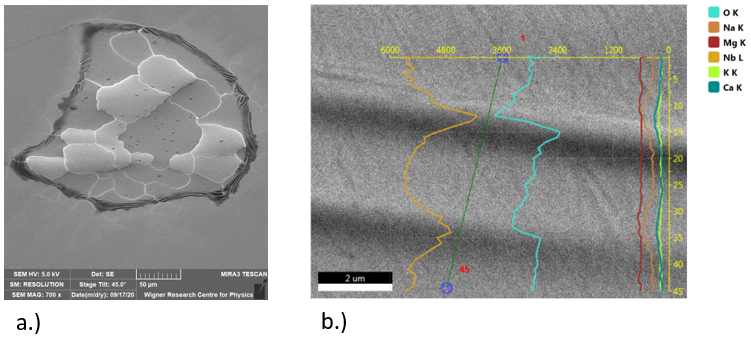
Formation of LiNbO3 nanocrystals by solvothermal method – An easy and relatively low temperature solvothermal (bottom-up) method was optimized for preparing LiNbO3 nanocrystals with homogenous particle size distribution 60-100 nm with a yield of 90 %. The effects of polyol media, reaction time and Li excess of the starting reagents were investigated. According to the X-ray diffraction phase analysis, Li3NbO4 and Nb2O5 have also been detected beside the LN phase in many samples depending on the ratio of the starting components and the reaction time. At 220 oC, the optimal synthesis parameters for the reaction of Nb2O5 with LiOH to form LiNbO3 were found to be diethylene glycol as polyol medium and 72-hour reaction time. Independently of the polyol medium used, the lithium oxide up-take of the niobium(V) oxide grains started with the formation of a lithium-rich phase, Li3NbO4, very likely at the Nb2O5/solvent boundary, which was followed by the equilibration of the concentrations within the grains. For the accomplishment of the LiNbO3 formation, a lithium oxide excess was necessary [1].
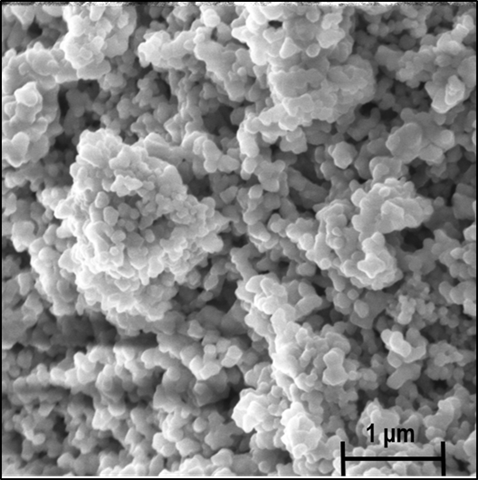
Figure 1. Typical SEM image of the LiNbO3 samples prepared by the solvothermal synthesis.
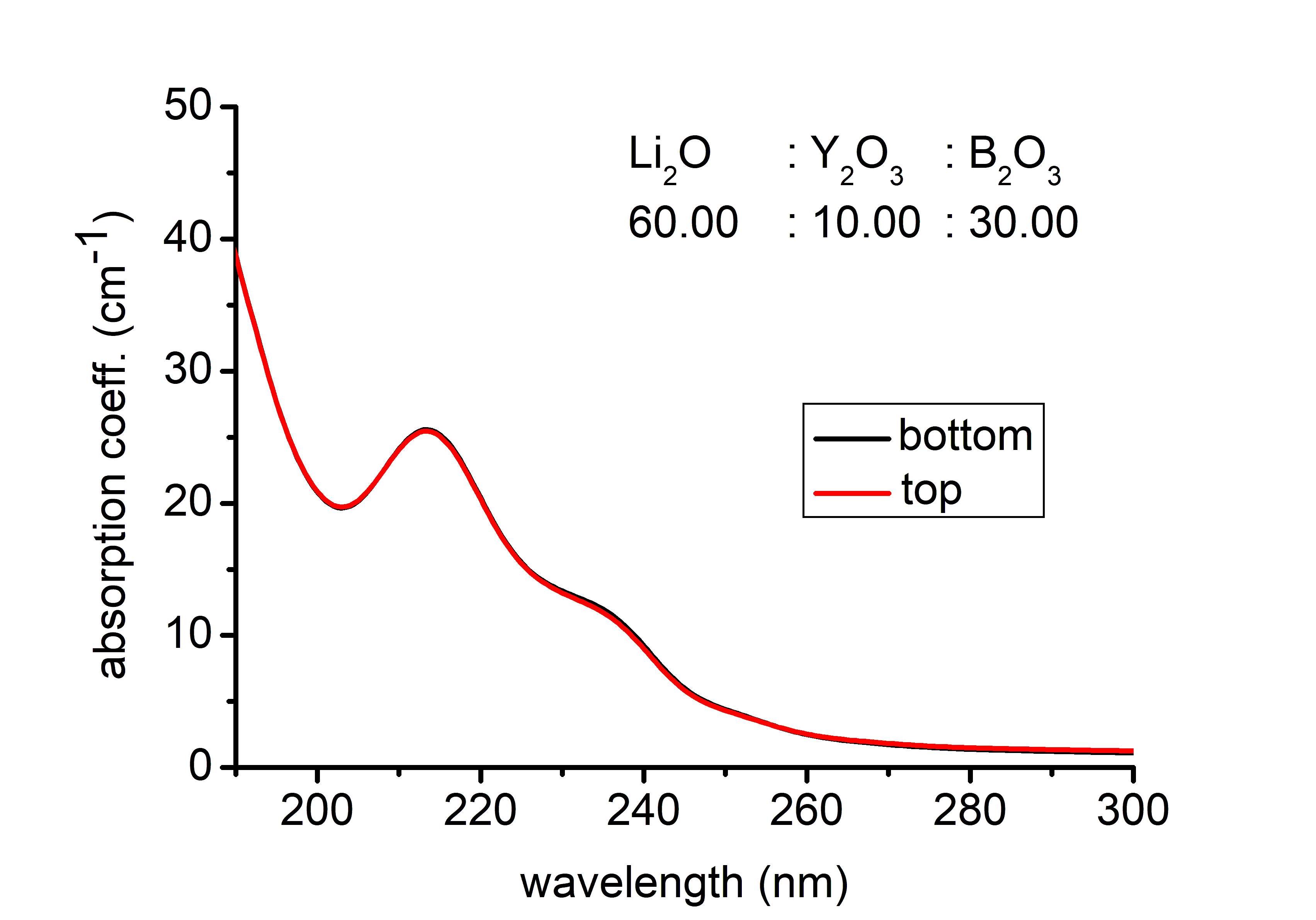
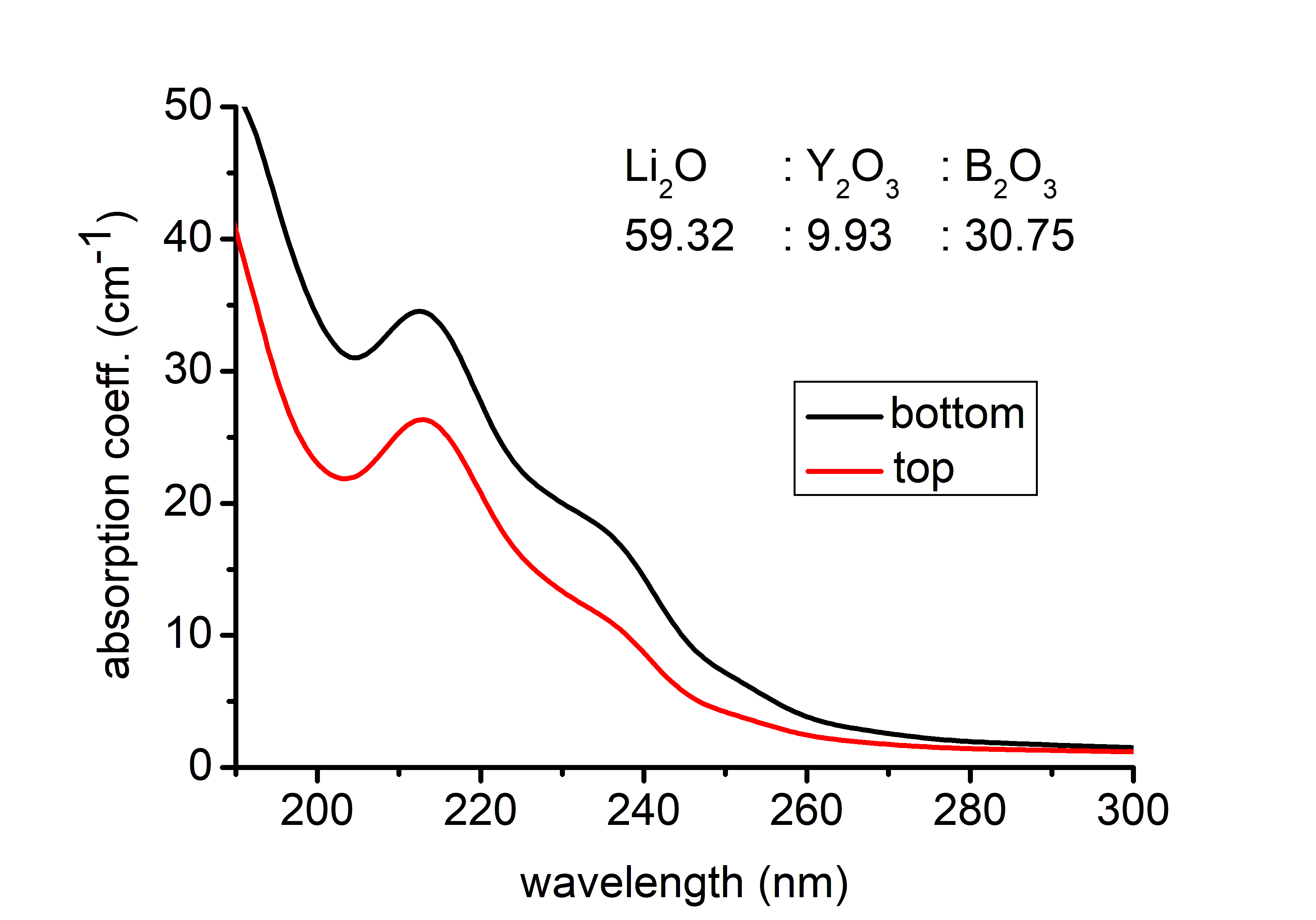
Preparation and investigation of LiNb1−xTaxO3 mixed crystals – Lithium niobate (LiNbO3, LN) and lithium tantalate (LiTaO3, LT) are uniaxial ferroelectric materials with high Curie temperatures and are characterized by a large spontaneous polarization. Due to their unusual large piezoelectric, electro-optic, and nonlinear coefficients they are employed for applications in linear and nonlinear integrated optics as well as surface acoustic wave generation. Because of the to the small ionic radius difference, Nb is easily substituted by the isoelectronic Ta, which allows for the fabrication of LiNb1−xTaxO3 mixed crystals over the complete compositional range creating the basis of the tunability of several physical composition dependent properties. Our goal was to produce homogeneous, crack- and inclusion-free monodomain crystals, to determine Ta/Nb in the axial and radial direction of the crystal and to investigate the composition-dependent spectroscopic properties. Mixed crystals were grown from the melt containing 5 mol% Ta. Due to the large difference in the melting points, between the LN seed and the melt containing 5 mol% Ta, cracks were visible at the beginning of the crystal. To prevent cracking, the seed of suitable composition was cut from the mixed crystal. After the next growth the crystal was successfully poled and turned to be monodomain. The Ta content was determined along with the growth (Y-cut) and the radial (Z-cut) axis with energy-dispersive X-ray spectrometry (EDS) in scanning electron microscope. The Ta content of the crystal decreases monotonically from top to bottom in the range of 15-10 mol%. The strongly differing optical absorption edge of congruent LN (320 nm) and LT (278 nm) may allow us also to follow the change of the [Ta]/[Nb] ratio in LiNb1−xTaxO3 mixed crystals. The absorption edge (wavelength at α = 20 cm-1) has shifted to longer wavelength along the growing axis from the top to the bottom part of the crystal indicating a slightly decreasing Ta content similarly to the results of EDS.
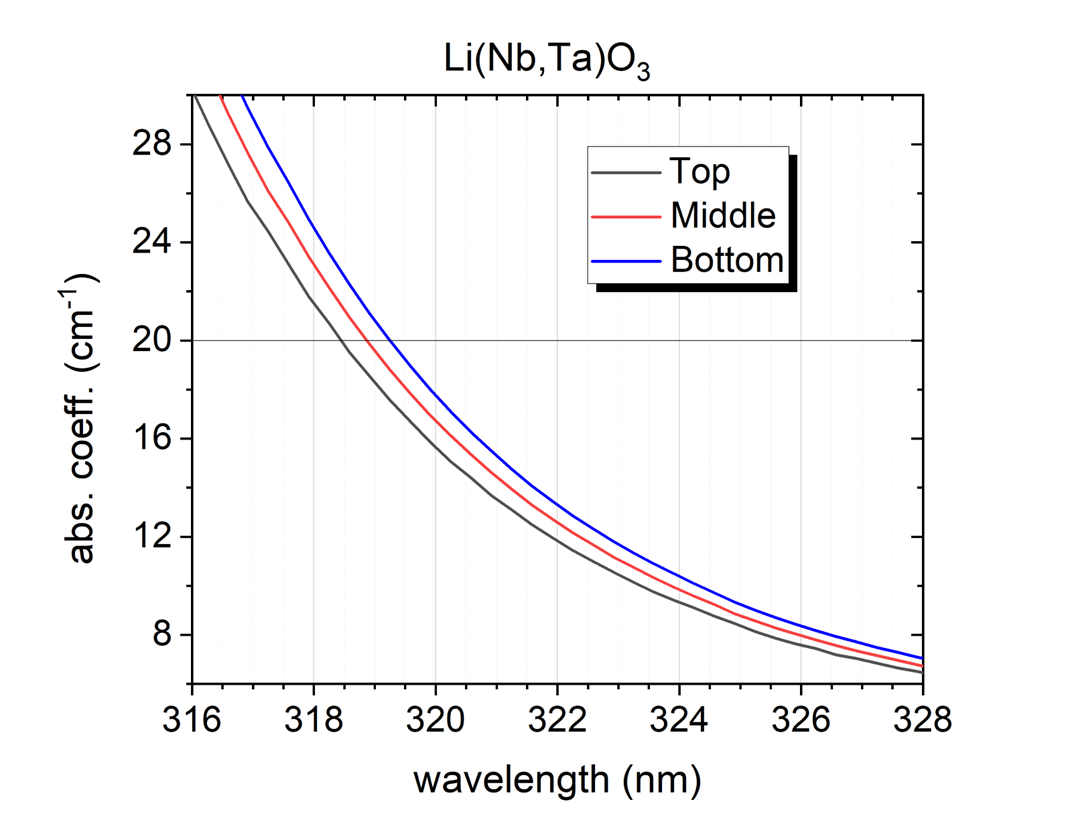
Figure 2. The absorption spectra of LiNb1−xTaxO3 sample plotted around the absorption edge to show the compositional change in the growing direction.
.
References
[1] G. Dravecz, T. Kolonits, L. Péter, Formation of LiNbO3 Nanocrystals Using the Solvothermal Method, Crystals 13:1, 77, 10 p. (2023)




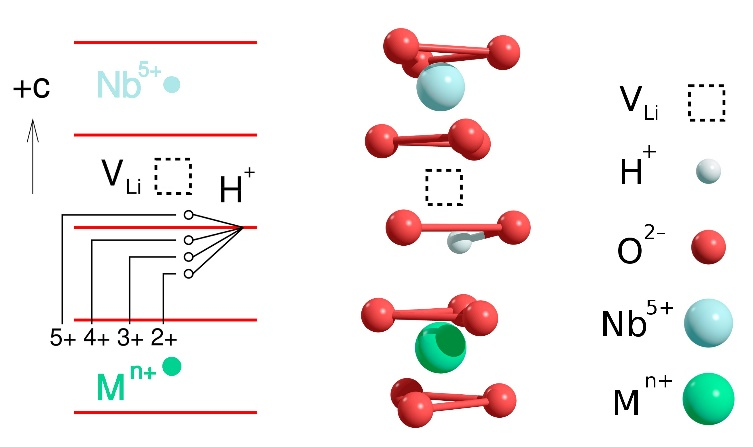
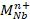 – OH type complexes, where the dopant M occupies a Nb site . The observed vibrational frequencies of the OH- ions and their polarization dependences agree well with the model established earlier for LiNbO3 doped with optical damage resistant and rare-earth ions, confirming its general validity (see Fig. 1). Hydroxyl ions are the most convenient probes for the detection of the change of any dopant incorporation from Li to Nb site at the threshold concentration
– OH type complexes, where the dopant M occupies a Nb site . The observed vibrational frequencies of the OH- ions and their polarization dependences agree well with the model established earlier for LiNbO3 doped with optical damage resistant and rare-earth ions, confirming its general validity (see Fig. 1). Hydroxyl ions are the most convenient probes for the detection of the change of any dopant incorporation from Li to Nb site at the threshold concentration